Health
Genetics & Genomics Research Dissemination; Makerere Bioethicists Emphasize the Importance of Community Engagement
Published
3 years agoon
By
Mak Editor
By Joseph Odoi
As Genetics research continues growing in Uganda, Bioethicists from Makerere University College of Health Sciences have stressed the importance of community engagement, genetic counselling and Public sensitization when conducting Genetics research in Uganda.
These recommendations were made at a research dissemination workshop held on the 8th December 2022 at Makerere University College of Health Sciences.
While presenting findings of the ELSI-UG project titled “Ethical and social issues in informed consentprocesses in African genomic research”, the Project Principal Investigator -Associate Professor Mwaka Erisa Sabakaki from College of Health Sciences, Makerere University in a special way welcomed participants to the dissemination. He noted that involving communities in genetics and genomics research is very important when it comes to enhancing the understanding of genetics and genomic information by the general public.
‘’There has been an exponential increase in genetics and genomic research in the last two decades.
However, this field of research is complex and is poorly understood by various research stakeholders. One way of enhancing understanding of genetics and genomic information by the general public is through community engagement. It is therefore crucial that communities are meaningfully involved in research processes right from conception. Community engagement provides a two-way communication channel through which researchers gain better understanding of community priorities, preferences, traditions, practices, and cultural sensitivities.’’ explained Prof. Mwaka.

He equally highlighted the need for translation of scientific language into local languages, genetic counsellors and consent in Genetics research adding that community engagement is crucial in building equitable research collaborations and trust between researchers and research communities.
Genetic and Genomics
According to National Institute of General Medical Sciences, Genetics is the scientific study of genes and how certain qualities, conditions or traits are passed from parents to their off springs. Genomics on the other hand involves using information about genes to: identify genetic disorders including future diseases so that doctors tailor treatment for individuals.
In same spirit, Dr. Moses Ochan, the Vice Chairperson of the Makerere University Research and Ethics Committee stressed the importance of sensitization of communities and researchers before any study is undertaken. According to him, sensitization enables communities understand the advantages and disadvantages of participating in a study thus making informed decisions.

In this United States National Institutes of Health funded study that sought to explore the knowledge,perceptions and experiences of stakeholders; researchers, bioethicists, REC members, research participants and caregivers/guardians on the informed consent process, and the ethical, legal and social implication of genomic research, 243 protocols were analyzed involving both local and international researchers
Findings
Return of individual genetic results to research participants
- Of 122 parents/caregivers of adolescents in the study, 77.1 % expressed the desire to receive all results of their children’s genetic/genomic results.
- 71.3 % of parents/caregivers agreed that children should be able to take part in research testing for genetic conditions that begin during childhood, even if there is no treatment that can alter the course of the condition
- 85.3 % of parents/ caregivers expressed the desire to know genetic research results about children to see if they are more likely to get a disease in the future.
- 71.3 % of parents/ caregivers agreed that Children should be able to take part in research testing for genetic conditions for which there is a treatment that begins during childhood that can alter the course of the condition
- 62.3 % of parents/ caregivers agreed that children should be able to take part in research testing for genetic conditions that start in adulthood and have no treatment that can alter the course
- 89.4 % of parents/ caregivers agreed that children should be able to take part in research testing for genetic conditions that will arise in their adult years, only if there is treatment or prevention that should begin in childhood

On the most important issues parents should consider in deciding whether or not to get genetic research results, 81.2% cited distress knowing that there are potential problems for other family members. Additionally, 45.0 % of parents and caregivers noted that receiving their child’s genetic results might worry their family; and 27.8% worried about stigma and discrimination
To address this, 69.2 % of parents and care givers said genetic counselling should be offered prior to a sample being taken to do genetic research
On perceptions on returning individual results of genomic research, parents and caregivers indicated that It is the researchers’ moral obligation to return clinically significant results; as such, genetic results should be communicated to them by the study doctor. Most parents preferred being informed first before involving the children; and some mothers expressed the desire to exclude the child’s father from these discussions until they (mothers) have understood the implications of the results in question.
On the role of children in making decision makings on whether to regarding return of genetic results or not, there was no consensus on the ideal age for disclosure of results. Some parents and caregivers pointed out that involvement of children in these discussions should depend on child’s character, level of understanding and ability to cope with the implications..
On handling findings that have familial implications, there were mixed feelings about involving other family members. Parents, especially mothers expressed fear of attribution. They thus suggested that the biological parents of the child should be the first ones to receive these results and then decide whether to involve other family members.
On the perceived challenges to return of results, parents and caregivers cited protracted delays in communicating genetics/genomics results; difficulty in tracing the child’s family, especially when the parents die and they are being cared for by other caregivers; risks of knowing unpleasant findings and paternity disputes.
Parents and caregivers offered several suggestions for the safe return of results of paediatric genomic research and these included the need to organize peer support and sensitization activities for adolescents participating in genetic studies; feedback of results should be done by a multidisciplinary team comprising of clinicians, genetic counsellors, the child and parents. All concurred that other family members should be involved at a later stage.
Informed consent and sharing of biological samples in collaborative genomic research and biobanking
On consent to future use of samples, 88.8% of the 187 researchers that participated in the study indicated that there is need to provide donors with the option to consent. 62% indicated that informed consent forms should include multiple options regarding the types and conditions of future research for which the samples may be used (tiered consent). 6.2% said that participants should only consent for the current study, and any future studies on the stored samples would require re-consent. However, the majority of researchers felt that the need to reconsent places an unacceptable burden on the researchers (62%) and is prohibitively costly (59.4%)
On informed consent experiences and practices, it was found that most principal investigators (12/15) were not well conversant with the informed consent procedures of their respective studies because they delegate this to study coordinators and nurses/nurse counsellors. Most nurses/nurse counsellors lacked basic knowledge and understanding of genetics, including the risks of genetic research.
On Information disclosure, researchers noted that genetic research is complex and oftentimes research participants do not adequately understand the information disclosed them during the consenting process. They thus recommended the use of an iterative approach that encourages consultation with family and/or people research participants trust, use of simple language, use of visual aids and other media, and objective assessment of comprehension. The also reiterated the need for translating informed consent documents into local languages and the use of peer educators. Researchers emphasized the role of community engagement in community education and sensitization, ensuring that researchers respect local cultural values and beliefs, and dispelling of superstitions and misinformation.
- The perceived challenges to the informed consent process included, the poor quality and inaccuracy of translations of ICF into local languages, inadequate understanding of informed consent, limited understanding of genetics by communities and some research team members, lack of professional genetic counselling services in Uganda, and mistrust of foreign collaborators.
On Export of human biological materials (HBM), researchers had a positive attitude towards the export of samples and expressed a desire for collaborative partnerships in genetics/genomic research and bio banking that are characterized by mutual respect and equity. However, they raised several concerns:
- They seem not to be well conversant with the guidance provided by the national ethics guidelines on bio banking and
- They all concurred that material transfer agreements (MTA) are key in the transfer of human biological materials across the national borders. However, they surmised that these MTA are unfair and tend to favour international Collaborators. They felt that local researchers and research institutions are not empowered enough to bargain favorably during MTA negotiations. They also indicated that the national ethics guidelines are vague on role of RECs in MTA and data sharing agreement development. Furthermore, they indicated that Uganda lacks appropriate enabling ethical and legal frameworks to protect the interests of local scientists and research institutions
- On sharing of the benefits of research, the researchers felt the ground was not leveled and there was neither equity nor fairness in sharing of GBR benefits in international collaborative research. They attributed this to the lack of scientific integrity and questionable research practices by collaborating researchers, lack of effective communication between collaborating partners, denial of access to shared data and samples by Northern collaborators, and felt that the oversight function of UNCST during MTA implementation is limited.

To address the issues at hand around genetics and genomics research, they made the following recommendations;
Recommendations to enhance comprehension of informed consent for genetic/genomic research and biobanking
- Escalating community engagement: to sensitize the general public and educate them on genetics research and its implications
- Iterative approach to informed consent where participants are given ample time to read/be read to consent information, ask questions, make consultations with family and trusted persons
- Encouraging the use of simple language and various media during information disclosure.
- There is need for harmonization of translations. A dictionary of translated key scientific and medical terms/concepts in research and clinical care in local languages should be developed
- Develop specific national guidelines for genetic and genomic research in Uganda.
- Research ethics committees should be trained in the basics of genetic research in order to ensure that they appreciate the ELSI and are competent enough to review genetic research.
- The use of checklists for assessing understanding of consent should become mandatory and should also be included in the national ethics guidelines.
- All stakeholders should read and understand the available national and international guidelines, policies, and regulations pertaining to genetics/genomic research and bio banking before negotiating Material transfer agreements.
- Research ethics committees should be empowered to review and monitor the execution of MTAs during research implementation, and this should be clearly stipulated in the national ethics guidelines.
- The national research regulators and individual institutions should join forces and devise mechanisms for tracking and monitoring the use of exported HBM and data.
- Encouraging meaningful involvement of communities in Material transfer agreements negotiations, particularly regarding sharing of the benefits of research.
- There should be capacity building for clinical genetics, particularly clinical geneticists and professional genetic counsellors
- Community engagement activities should be scaled up to prepare communities for the return of genetic research results as and when they are available
More about the Project
This project explored the knowledge, perceptions and experiences of stakeholders on the informed consent process, and the ethical, legal and social implication of genomic research. The goal of the project was to contribute to a better understanding of the ethical legal and societal issues associated with genomic research in low resource settings. The study employed both quantitative and qualitative methods of data collection and analysis. Prospective evaluation was done using questionnaire surveys; focus group discussions; in-depth interviews; direct observation of informed consent processes; and assessment of the quality of informed consent
This study was funded by United States National Institutes of Health through The Human Heredity and
Health in Africa (H3Africa) initiative which is spearheading bio banking and genomics research in Africa for Africa.
The study was conducted between November 2018 to 2022 by a team of researchers led by Associate Prof. Erisa Mwaka as Principal Investigator.
Research team:
- Associate Prof. Erisa Mwaka
- Dr. Ian Munabi
- Assoc. Prof. Joseph Ochieng
- Dr. Janet Nakigudde
- Prof. Nelson Sewankambo
You may like
-


76th Graduation Highlights
-

Mak hosts First African Symposium on Natural Capital Accounting and Climate-Sensitive Macroeconomic Modelling
-


Strengthening Global Partnerships to Advance Research, Innovation, and Graduate Training: Makerere University Hosts Delegation from the University of Warwick
-


Mastercard Foundation Scholars embrace and honour their rich cultural diversity
-


First African Symposium underscores the role of the Centre of Excellence for Africa Climate-Sensitive Macroeconomic Modelling
-


Makerere Hosts Second Cohort of MoKCC&MA Procurement Officers for E&S Safeguards Training
Health
Makerere University School of Public Health Graduates First Cohort of Cost-Effectiveness Analysis Short Course
Published
4 days agoon
February 20, 2026By
Mak Editor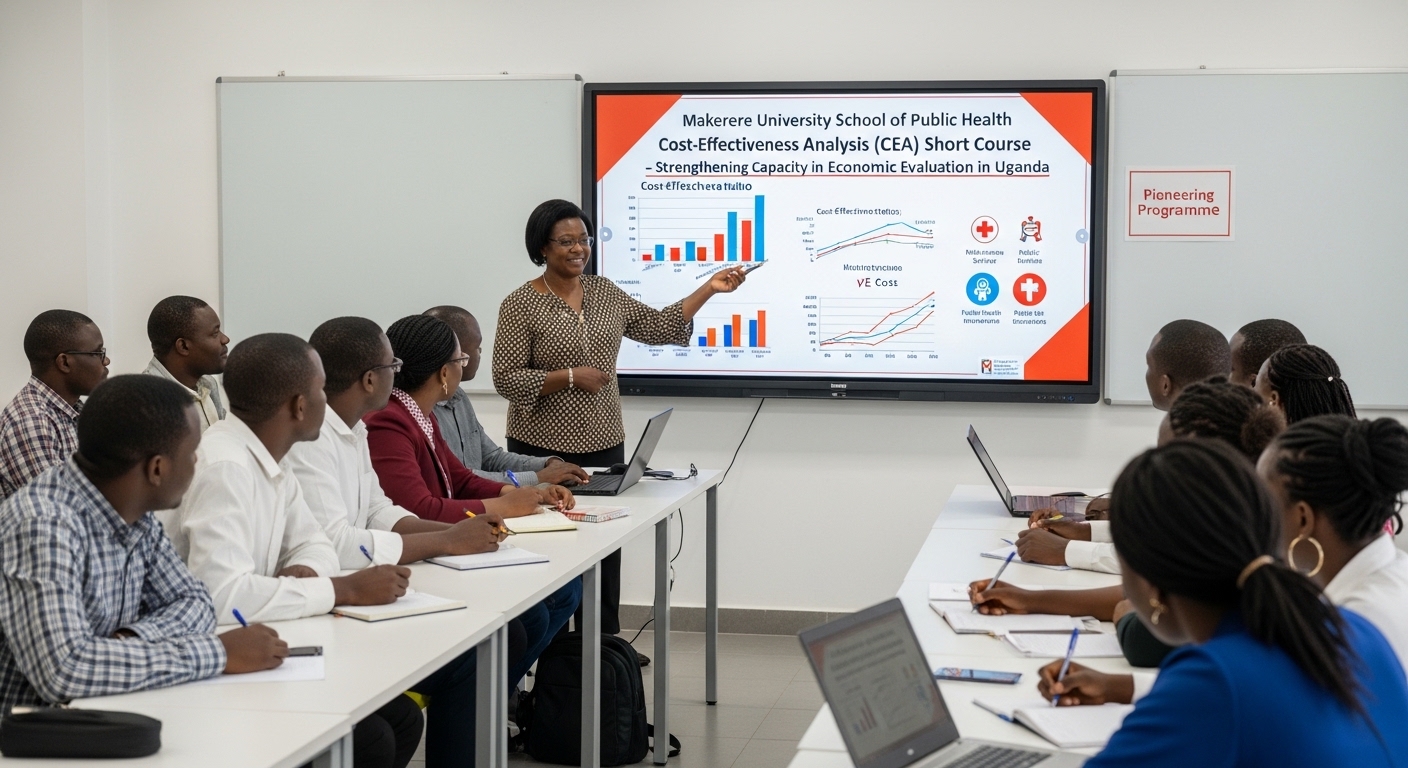
Kampala, Uganda – The Makerere University School of Public Health (MakSPH) has marked a significant milestone with the graduation of the first-ever cohort of its Cost-Effectiveness Analysis (CEA) Short Course. The pioneering programme is designed to strengthen capacity in economic evaluation in Uganda and beyond.
The virtual graduation ceremony honored eleven (11) participants who completed the course. The cohort included professionals from academia, research institutions, government agencies, and non-state actors, reflecting the increasing demand for skills in economic evaluation across sectors.
The short course was developed and implemented by the Department of Health Policy, Planning, and Management (HPPM) in response to the increasing need for evidence-informed decision-making in a context of limited resources.
In her remarks during the ceremony, Assoc. Prof. Suzanne Kiwanuka, Head of the Department of Health Policy, Planning and Management (HPPM) at MakSPH, congratulated the inaugural cohort for completing what she described as a “critical and timely” course.
“With decreasing resources and rising demand for services driven by population growth and the emergence of high-cost technologies, decision-makers must make difficult choices,” she noted. “Cost-effectiveness analysis is no longer optional. It is central to conversations in the corridors of power.”
The CEA short course was designed to equip policymakers, researchers, and practitioners with both theoretical knowledge and practical skills in economic evaluation. Participants were introduced to key principles of health economics, costing methodologies, decision-analytic modelling, Markov modelling, sensitivity analysis, and interpretation of incremental cost-effectiveness ratios (ICERs).
According to Prof. Elizabeth Ekirapa, the course lead at MakSPH, this inaugural offering had been “a long time coming,” following years of discussions within the department about building local expertise in economic evaluation.
Delivered over 10 days through interactive online sessions, the course combined lectures, case studies, and hands-on modelling exercises using contextually relevant datasets. Participants were required to develop and present applied cost-effectiveness projects as part of their assessment, allowing them to translate theory into practice.

During the feedback session at the graduation ceremony, faculty emphasized the importance of clarity in defining study perspectives, selecting appropriate outcomes, and aligning research questions with modelling approaches.
Dr. Chrispus Mayora, one of the facilitators, highlighted the need to carefully select outcomes that directly reflect the intervention being evaluated. “When thinking about outcomes, ask yourself: Is this aligned with what I want to study? Interesting outcomes are not always the most appropriate ones,” he advised.
Participants were also encouraged to select modelling techniques such as decision trees or Markov models based on the research question and the nature of the disease or intervention under study.
Prof. Ekirapa described the graduates as “trailblazers,” noting that their feedback would shape future iterations of the course. “When you are the first cohort, you are like pioneers,” she remarked. “We are committed to improving this course to ensure it becomes a world-class programme.”
For many attendees, the graduation ceremony was a new experience, as certificates were awarded virtually an approach that participants welcomed as innovative and inclusive.
“Cost-effectiveness analysis enables us to maximise value for money,” noted Dr. Crispus Mayora of MakSPH. “It allows decision-makers to compare interventions systematically and ensure that limited resources achieve the greatest possible benefit.”
The programme aligns with Makerere University’s broader mandate to provide high-quality training that responds to national and regional development priorities. Participants who successfully complete the course receive a certificate signed by the Dean of the School of Public Health.
As the ceremony concluded, faculty encouraged continued engagement beyond the classroom. Graduates were urged to refine their project ideas and collaborate with the department in advancing research and policy discussions.
The successful completion of the first CEA short course marks an important step in building a cadre of professionals equipped to conduct rigorous economic evaluations. With plans to expand and refine the programme based on participant feedback, the HPPM department under MakSPH is positioning itself as a regional leader in health economics and policy analysis training.
Health
Uganda has until 2030 to end Open Defecation as Ntaro’s PhD Examines Kabale’s Progress
Published
6 days agoon
February 18, 2026
Silhouettes slip along narrow paths, farmers heading to their gardens, women balancing yellow jerrycans on their hips, children in oversized sweaters hurrying to school, and herders steering cattle toward open pasture, each movement part of a choreography older than memory. This is a quiet ritual in Kabale’s terraced hills, moments before the sun lifts.
The quiet procession to ahakashaka, or omukishaka, often sees figures moving quickly along familiar footpaths in the half-light, as children and adults walk with the urgency of habit. It is not a stroll but often a small, hurried run before daylight exposes what should be private.
It is February 2026, and the century-old Makerere University is celebrating its 76th Graduation Ceremony. The world paces and races toward artificial intelligence and digital revolutions. But some families still begin their day by rushing to the bushes for relief and concealment, while others engaged in economic activities such as gardening and grazing have no sanitation option other than using their surroundings to respond to the nature call!
The deadline to end open defecation is 2030. The science is settled, and the commitments are written into Sustainable Development Goal 6. Yet in parts of Kabale, only a small fraction of households is truly open defecation free.
In his PhD research, Dr. Moses Ntaro did not start with global targets or conference declarations. He began where the morning run ends, at the edge of the compounds, behind banana stems, along worn paths leading to Omukishaka. He asked whether students, equipped not with bricks but with conversation, follow-up, and persistence, could help communities replace that dash with something quieter: a door that closes.
What he found is both hopeful and unsettling. Change is possible. But dignity, like sunrise, should not require a run. And with 2030 approaching, time is no longer generous.

The Question That Would Not Let Him Go
Ntaro did not encounter open defecation as a statistic. While on foot and serving as Assistant Coordinator of Community-Based Education at Mbarara University of Science and Technology (MUST), he learned while supervising students placed in rural communities across southwestern Uganda. They walked villages together, conducted transect walks… and they observed.
“In my role as academic coordinator,” he explains, “students always took me on transect walks within the villages to show me how high open defecation practice was. The effect was evident in the high prevalence of intestinal infections we saw in health facility records.”
The link between sanitation and disease was not theoretical but visible in clinic registers. Diarrhea, intestinal worms, recurring infections among children, and more were all visible in the clinic registers.
Nineteen years ago, in 2007, Uganda adopted Community-Led Total Sanitation (CLTS), a strategy designed to trigger collective behavior change and eliminate open defecation. Progress, however, remained uneven. That same year, Ntaro was working as an Environmental Health Officer with the Water and Sanitation Development Facility under the Ministry of Water and Environment. He was three years away from completing his Environmental Health degree at Makerere University School of Public Health.
And so, the question emerged, to Ntaro, that, ‘If students are already embedded in these communities through COBERS placements, why are we not intentionally harnessing them to accelerate sanitation change?’
That question became his PhD.

This is a Crisis That Should No Longer Exist
Globally, more than 350 million people still practice open defecation. Sub-Saharan Africa carries a disproportionate share. SDG 6, specifically Target 6.2, commits the world to ending open defecation and ensuring universal access to safe sanitation and hygiene by 2030. It prioritizes women, girls, and vulnerable populations. It speaks of dignity, of safely managed services, and of disease prevention.
We are four years away from that deadline. And in rural Kabale District, somewhere in southwestern Uganda, Ntaro’s research found that only 3 percent of households were truly open defecation-free.
Yes, three percent. His 2025 BMC Public Health study examined 492 residents. The average age was 49. Nearly 30 percent had no formal education. Most were women, the custodians of household hygiene and child health.
The determinants of Open Defecation Free (ODF) status were deeply behavioral.
Male-headed households had higher odds of being ODF. Households with clean compounds, clean latrine holes, and consistent handwashing practices were significantly more likely to sustain sanitation improvements.
Sanitation, Ntaro realized, is not only infrastructure but also power, memory, habit, and social expectation.
“Factors associated with ODF status were not just economic,” he notes. “They were behavioral and contextual.”

Why It Feels So Wrong to Still Discuss This
Talking about open defecation in 2026 feels unsettling for three reasons. First, it feels like a failure of basic dignity.
Think of an era of global connectivity and rapid technological advancement, and hundreds of millions still lack privacy. For women and girls, this exposes them to harassment, exploitation, and fear. Sanitation is not just about disease but safety.
Second, it feels like an avoidable health crisis. One gram of feces can contain millions of viruses, bacteria, and parasites. Open defecation directly fuels cholera, typhoid, diarrhea, and environmental enteropathy, a silent contributor to child malnutrition and stunting. The science is settled, and yet the practice persists.
Third, it feels like a poverty trap. Illness leads to lost productivity; lost productivity deepens poverty, and poverty limits investment in sanitation. The cycle continues.
“Open defecation is not simply a sanitation issue,” Ntaro says. “It is linked to poverty, nutrition, and broader development.”

Testing a Different Approach
Ntaro’s doctoral thesis, “Effect of Student Community Engagement on Open Defecation-Free Status,” tested whether health profession students could effectively facilitate Community-Led Total Sanitation.
In some villages, traditional Health Extension Workers led the sanitation process. In others, trained students facilitated it under the COBERS (Community-Based Education, Research, and Service) model, which places medical trainees in community health facilities to learn through real-world practice, bridging classroom theory with primary care and public health work in rural settings.
Through this model, students led triggering, follow-ups, and community engagement. Open defecation declined. More households achieved Open Defecation Free status. And the cost per household was lower than in traditional approaches.
“Students were more effective,” Ntaro explains. “More households became open defecation-free compared to the traditional approach. And they were a cheaper human resource.”
But cost was not the real breakthrough. Presence was. Students stayed for weeks. They returned to check on latrines. They built trust. They kept coming back. Because sustainability, Ntaro argues, is not built in a single visit. It is built in repetition.
“There is a need for continued follow-ups and continued student engagement if long-term impact is to be realized.”
Change cannot be declared once and forgotten.

Behavior… and Not Just Bricks
Using the RANAS framework, Ntaro found that households that remembered to wash hands and kept latrines clean were far more likely to sustain Open Defecation Free status. In sanitation, behavior leaves evidence.
“Behavioral change interventions that empower communities,” he recommends, “such as CLTSH, should be strengthened to increase households with ODF status.”
In other words, building latrines is not enough, but communities must believe in them.

The Defense and the Countdown
On December 11, 2025, Ntaro defended his PhD. Examiners pressed him on scale and sustainability. Could student engagement be institutionalized? Could universities be embedded in district sanitation planning?
His answer was pragmatic: “Yes, but community-based education must be included in planning and budgeting.”
Four years remain to meet SDG 6.2. Four years to end open defecation and turn dignity from promise into practice. In 2026, this conversation should feel outdated. Instead, it remains urgent.

The Slow Work of Restoration
In Kabale, progress does not look dramatic. It looks like a latrine door closing firmly behind someone, a handwashing station with water and soap, a compound swept clean. It looks like a child who does not fall ill this month. Public health victories are often quiet.
As Makerere University approaches its 76th Graduation Ceremony, Dr. Ntaro Moses stands among its PhD graduands not with theory alone, but with evidence that change can be accelerated by reimagining who leads it. Students, he shows, are not only learners. They are the workforce, facilitators, and bridges between policy and path.
The hills of Kabale still wake under mist. But in more compounds now, privacy exists where bushes once stood open. Dignity is not restored in headlines, but one household at a time.
And with 2030 approaching, Ntaro’s work leaves a final, unavoidable question: if we already know how to end open defecation, if we already have the tools, the evidence, and the people, what, exactly, are we waiting for?

— Makerere University School of Public Health Communications Office, Graduation Profiles Series, 76th Graduation Ceremony
Health
Olivia Nakisita and the Quiet Urgency of Adolescent Refugee Health
Published
6 days agoon
February 18, 2026
Kampala wakes early, but for some girls, the day begins already heavy. In Uganda, nearly three-quarters of the population is under 30, growing up happens fast, and often without protection. One in four Ugandan girls aged 15–19 has already begun childbearing, giving Uganda the highest teenage pregnancy rate in East Africa.
Layered onto this is displacement. The country hosts about 1.7 million refugees, many living in cities like Kampala, where survival depends on navigating systems not designed with them in mind. Also, nationally, 1.4 million people live with HIV, and 70 per cent of new infections among young people occur in adolescent girls, a reminder that vulnerability is rarely singular. When COVID-19 shut the country down, the consequences were immediate, with pregnancies among girls aged 15–19 rising by 25.5 per cent, while pregnancies among girls aged 10–14 surged by 366 per cent.
The numbers tell a story of youth, risk, and quiet urgency. But they do not tell it all. For years, Olivia Nakisita, a public health researcher,has followed how adolescent girls, many of them refugees, navigate pregnancy in Kampala: how far they must travel for care, how early they arrive or delay, and how often services that exist fail to meet them where they are. Her work lives at the uneasy intersection of policy and lived reality, where access does not always translate into care.
February 25th 2026, is the day that her work on whether urban health systems are truly ready for the youngest mothers they now serve will bring her to Freedom Square at Makerere University, where she will graduate with a PhD in Public Health.

Her doctoral journey, focused on maternal health services for adolescent refugees in urban Uganda, has unfolded at the intersection of scholarship, community service, and the daily realities of young girls navigating pregnancy far from home.
The Work That Came Before the Question
Long before she began writing a PhD proposal, Olivia Nakisita was already immersed in adolescent health. As a Research Associate in the Department of Community Health and Behavioral Sciences at Makerere University’s School of Public Health, she taught graduate and undergraduate students, supervised Master’s research, and worked closely with communities. Beyond the university, she led New Life Adolescent and Youth Organization (NAYO), a women-led organisation she founded in 2021 to strengthen access to sexual and reproductive health and rights (SRHR) information and services for adolescents and young people.
It was through this community work that a troubling pattern began to surface.
“During our community service,” she explains, “we noted increasing teenage pregnancies, and we also noted challenges with access to maternal health services by teenage pregnant girls.”

Among those girls were adolescents living as urban refugees in Kampala, young, displaced, often poor, and navigating pregnancy in a city not designed with them in mind.
For Nakisita, the concern deepened through her academic training in Public Health Disaster Management, one such programme that prepares multidisciplinary professionals with the technical expertise and leadership competencies required to plan for, mitigate, respond to, and recover from complex disasters through a public health lens. This programme sharpened Nakisita’s interest in how displaced populations survive within complex urban systems. Uganda’s integrated health model, where refugees and host communities are expected to use the same facilities, appears equitable on paper. In practice, it can be unforgiving.
“I got interested in understanding how these refugees who get pregnant manage to navigate the complexities of integration in host societies like Kampala,” she says. “This was driven by the desire to address their needs and to inform and evaluate existing refugee health policies.”

That desire became the foundation of her PhD.
Asking Hard Questions in a Crowded City
Her doctoral research, “Maternal Health Services for Adolescent Refugees in Urban Settings in Uganda: Access, Utilisation, and Health Facility Readiness,” was conducted in Kampala between November 2023 and August 2024. It combined quantitative surveys with qualitative interviews, engaging 637 adolescent refugees aged 10–19 years, alongside health workers and facility assessments.
Her findings showed high perceived access to maternal health services. Clinics existed. Services were available. Yet utilisation, particularly of antenatal care (ANC), lagged. “About three-quarters of the girls attended at least one antenatal visit,” she explains, “but only about four in ten attended in the first trimester.”
And that gap matters. Public health research shows that early and regular antenatal care allows health workers to detect high-risk pregnancies, initiate supplements such as iron and folic acid, monitor fetal development, and provide psychosocial support. Without it, risks compound silently.
By contrast, her study found that facility-based deliveries were remarkably high, with nearly all adolescent refugees (98.3%) giving birth in health facilities, suggesting that the system was reachable, but uneven.

Where the System Falls Short
Her research went beyond utilisation to examine whether health facilities were actually ready to serve adolescent refugees.
Findings show that lower-level health centres in Kampala were moderately prepared to offer adolescent-friendly maternal health services. Some staff were trained. Some spaces existed. Despite this, critical gaps remained. For instance, facilities lacked essential equipment and supplies. Non-provider staff were often untrained. Separate, private spaces for adolescents were limited. Language barriers complicated care. Overcrowding strained already stretched health workers.
In her qualitative interviews, health workers expressed empathy and willingness to help. Many relied on peer educators and community health workers to reach adolescent refugees. But good intentions were not enough.
“They recommended training of healthcare workers, translators for refugees, and improvement in the availability of essential drugs, supplies, and equipment,” Nakisita notes.
She notes that readiness is not just about infrastructure but about the people, preparation, and priorities.
Research with an Emotional Cost
For Nakisita, working with adolescent refugees required care, not only methodologically, but emotionally.
Finding participants in Kampala was itself a challenge. Unlike settlement settings, urban refugees are dispersed, often invisible. Ethical considerations were constant. Adolescents who had given birth were legally considered emancipated minors, but their vulnerability remained.
Though the thesis focused on systems rather than personal narratives, Nakisita’s earlier work with adolescents informed every decision she made. It shaped how she framed questions, interpreted data, and weighed policy implications. This was not detached research, but careful, deliberate, and grounded.
The Scholar Formed by Continuity
Nakisita’s PhD sits atop more than 18 years of experience in training, research, and community service. She is an alumna of Makerere College School (UCE), 1996 and Greenhill Academy Secondary School (UACE), 1998, a long journey through Uganda’s education system before her Diploma in Project Planning and Management at Makerere University completed in early 2000s.
She would later return eight years later to Makerere University for her Bachelor’s degree in Social Sciences and a Master’s in Public Health Disaster Management, and now a PhD in Public Health.
Her academic rigor is reflected in extensive training across SRHR, impact evaluation, research methods, ethics, disaster resilience, and humanitarian health. She has presented at regional and international conferences and published in peer-reviewed journals on adolescent health, refugee maternal care, gender-based violence, and health systems readiness.
As a PhD student, she supervised three Master’s students to completion, with another currently progressing, quietly extending her influence through mentorship.



When Evidence Demands Action
If policymakers were to act on one lesson from her research, Nakisita says; “Emphasis should be given to maternal health services for adolescents.” “They are high-risk mothers,” she adds.
Her findings call for targeted community-based interventions, outreaches, home visits, and financial support for adolescents who cannot afford prescribed drugs, delivery requirements, or critical tests like ultrasound scans.
They also call for health systems to move beyond one-size-fits-all models, recognising that age, displacement, and poverty intersect to shape how care is accessed and experienced.
Now that her PhD is complete, Nakisita plans to translate research into action. Several papers from her study have already been published. A policy brief is planned to influence decision-making in urban and humanitarian health settings.
When asked what she would say directly to adolescent refugee girls navigating pregnancy in unfamiliar cities, her response is simple and direct.
“If it happens,” she says, “as soon as you find out, go to the nearest health facility and seek care. Always return for the visits as asked by the health worker. Ensure that you deliver in a health facility with a skilled health worker.”
Arrival, Without Illusion
When Dr. Olivia Nakisita steps onto the graduation stage at Freedom Square, applause will follow. But the true significance of that moment lies in health facilities still struggling to adapt; in adolescent refugees whose pregnancies unfold quietly in rented rooms and crowded neighborhoods; in policies waiting to be sharpened by evidence.
Her scholarship does not promise quick fixes but offers clarity.
Among the PhDs conferred at Makerere University’s 76th graduation, her work reminds us that some research does not begin in libraries and does not end with theses. It lives on in the slow, necessary work of making health systems see those they have long overlooked.
— Makerere University School of Public Health Communications Office, Graduation Profiles Series, 76th Graduation Ceremony
Trending
-

 Humanities & Social Sciences2 days ago
Humanities & Social Sciences2 days agoMeet Najjuka Whitney, The Girl Who Missed Law and Found Her Voice
-

 Health6 days ago
Health6 days agoUganda has until 2030 to end Open Defecation as Ntaro’s PhD Examines Kabale’s Progress
-

 Agriculture & Environment5 days ago
Agriculture & Environment5 days agoUganda Martyrs Namugongo Students Turn Organic Waste into Soap in an Innovative School Project on Sustainable Waste Management
-

 General6 days ago
General6 days agoMastercard Foundation Scholars embrace and honour their rich cultural diversity
-

 Health2 weeks ago
Health2 weeks agoCall for Applications: Short Course in Molecular Diagnostics March 2026
