Health
CTCA Job Advert 3 Positions: Clinical Trial & Training Coordinator, Data Manager, Project Admin.
Published
2 years agoon
The Centre for Tobacco Control in Africa (CTCA), is a constituent entity of Makerere University and the School of Public Health responsible for capacity building and research for tobacco control in Africa. CTCA in collaboration with the University of Southern California (USC) are implementing a 5-year research project titled “Quit4Life+: Adapting and Evaluating a Phone-Based Tobacco Use Cessation Program for People Living with HIV in Uganda and Zambia”. This randomized controlled trial aims to promote tobacco cessation among HIV infected persons through adapting a standard short message service (SMS) intervention tailored to meet the needs of PLWH (Quit4Life+) for tobacco cessation, and determining the efficacy of the SMS-based intervention through a randomized trial with current standard of care as the control, in Uganda and Zambia.
The study will provide insights into the efficacy, feasibility, and applicability of delivering tobacco cessation interventions by health care professionals at HIV treatment centers in two countries with different tobacco use patterns, policy environments, and health care resources and provide needed information to health care providers and policymakers looking for cost-effective tobacco cessation interventions to inform scaleup of tobacco use cessation in LMICs worldwide. The project is therefore targeting professionals with experience in training and exposure to health field to fill the following positions;
Clinical Trial and Training Coordinator
Reports Directly to: The Principal Investigator Quite4Life Project at CTCA.
Directly Supervises: None
Duty Station: Kampala, Uganda
The position holder is expected to facilitate and participate in training for qualitative data collection, piloting stage of the study happening in October 2022 and at the randomized trial stage and is expected to start work in October 2022. This is a specific, time-bound contract type of assignment and not full-time employment.
Job purpose
The objective of recruiting a Clinical Trial and Training Coordinator is to prepare the research assistants, interviewers, data collectors, data entrants to understand the requirements of the task to enable the perform and deliver quality work. Therefore, the project seeks to recruit a professional responsible for holding the research program training component to realize project goal and specific. The Training Coordinator will be contracted for specific segments covering October 2022 to April 2023.
Duties and Responsibilities
- Conduct a study-wide training needs assessment and identify skills or knowledge gaps that need to be addressed
- Development of the training plan that will cover direct training, mentorship, and training evaluation.
- Design, prepare and order training aids and materials
- Select appropriate training methods or activities such as simulations, mentoring, on-the-job training, professional development classes
- Participate in review and development of materials, protocols, training manuals
- Participate in development of annual work-plans addressing training and mentorship/research exchange needs for the collaboration.
- Plan, coordinate and monitor trainee placement and how they fit in the study sites health service delivery
- Assess instructional effectiveness and determine the impact of training on research assistants’ skills and key performance indicators.
- Gather feedback from trainers and trainees after each educational session
- Partner with internal stakeholders and liaise with experts regarding instructional design
- Manage and maintain in-house training facilities and equipment
- Develop training reports
- Other roles will include but not limited to
- Ensure quality of the training processes and outputs
- Check on deadlines of the training activities
- Attend meetings
Qualifications
- Proven work experience as a Training Coordinator, Trainer, Training Facilitator or similar role
- Extensive knowledge of instructional design theory and implementation
- Adequate knowledge of learning management systems and web delivery tools
- A bachelor’s degree in Medicine, plus a master’s degree in Public Health
- Have excellent communication skills, both oral and writing skills.
- Proven ability to complete full training cycle:- assess needs, plan, develop, coordinate, monitor and evaluate i.e Possess training skills and experience necessary to analyse training.
- At least 2 years’ hands-on experience coordinating multiple training events in a research setting particularly RCTs
- Experience coordinating multi-site /country programs reporting, and collaboration program management will be an added advantage.
- Should have advanced computer skills, particularly statistical packages.
- Experience with e-learning platforms
Data Manager
Reports Directly to: The Principal Investigator Quite4Life Project at CTCA through the CO-I, Leading Statistics and Data Management.
Directly Supervises: None
Duty Station: Kampala, Uganda
Job purpose
The objective of recruiting a Data Manager is to manage the quantitative data collected. Therefore, the project seeks to recruit a professional who will be responsible for managing the study data.
The Data Manager will;
- Participate in the design of the data collection tools, data collection, and analysis
- Take lead in the design of electronic data entry/capture files/ formats
- Process data collection, and completing data collection tools
- Take lead the development of data collection and data management standard operating procedures
- Prepare regular checks on study data to help project management teams monitor data flows and data quality issues during the conduct of a study.
- Ensure accuracy, accessibility and data security and confidentiality, and storage of study data files and subject records.
- Participate in the preparation and execution of dissemination activities. These will include the preparation of technical reports, publications, blogs, PowerPoint presentations and engaging in-country stakeholders in dissemination activities.
- Participate in manuscript writing.
- Participate in meetings
- Execute any other data management related duties that may be assigned from time to time.
Qualifications, Skills and Experience:
Suitable applicants MUST possess
- A master’s degree in either Biostatistics, Epidemiology and Statistics, or equivalent degrees.
- At least two years of demonstrated relevant experience in managing health-related project research data, for projects of similar size and design.
- Demonstrated statistical programming skills in statistical software and database management particularly using STATA, R and other any other relevant software.
- Demonstrated experience in analytical skills and data management for projects of similar size and design.
- Ability to work independently with minimal supervision, strong interpersonal communication, and ability to work with diverse sectors as well as meeting deadlines.
- Ability to process, analyse, and present study results in a quality publishable format.
- Experience in database design and data management.
Project Administrator
Reports Directly to: The Principal Investigator Quite4Life Project at CTCA
Directly Supervises: None
Duty Station: Kampala, Uganda
Job purpose
The objective of recruiting a Project Administrator is to support the administrative components of the study to realize the project goal. The project administrator will be contracted for specific segments covering October 2022 to September 2023, and is renewable upon satisfactory performance. The Project Administrator will be required at dedicate 50% of their time on the project.
Duties and Responsibilities
- Work with the team to plan and track administration work for the Quit4Life+ project
- Organize project events, liaise with delegates, venues and trainers as required
- Perform clerical duties including typing, photocopying, scanning, faxing, filing, and mailing
- Assist project leads in the development of logistics plans for meetings, trainings, field activities and workshops • Assist respective project leads in drafting and distributing letters; and seek confirmation of participation for events organized by Quit4Life+ project
- Coordinating and scheduling conferences, meetings, and travel arrangements for traveling within and outside of Uganda
- Determine needs and coordinate the procurement of office supplies, equipment, repair and maintenance services.
- Ensure timely settlement of vendor payments (internet, transport, office rent etc.)
- Monitor monthly project expenditure and compile a quarterly budget request
- Support finance department with invoicing and expense tracking
- Coordinate with accounts for the submission of complete and accurate financial report
- Any other duties as assigned by the Principal Investigator
Qualifications and Attributes
- Degree in business administration with a bias in either or accounts, finance, and administration, and any other related field.
- At least 1 years’ experience in administrative work
- Excellent verbal and written English language skills
- Financial management skills
- Exceptional organizational skills and attention to detail
- Proven capacity to take initiative and willingness to learn new skills as needed
- Strong work ethic and the ability to work well independently and as part of a team
- Outstanding interpersonal skills and ability to interact with individuals at all levels including the ability to communicate in an effective manner with a wide range of stakeholders
How to apply
i) Qualified and interested candidates are invited to submit their application documents and a motivation letter clearly highlighting the position being applied for and address this to;
The Dean,
Makerere University School of Public Health,
College of Health Sciences, Makerere University,
P.O. Box 7072, Kampala, Uganda
ii) Application Documents
a) Motivational Letter
b) Resume with contacts of 3 professional referees
c) Copies of all relevant academic documents
iii) Soft copies of the applications should be submitted as one PDF file to the following email address EOI@ctc-africa.org by 5:00pm on Wednesday, September 7, 2022. Please quote the position you are applying for in the subject head of your email.
You may like
-


Building Resilience: Makerere Leads Climate Finance Training for Finance Officials
-


FoodLAND Project Research Dissemination: Nakaseke District Farmers Sensitized on Modern Agricultural Practices & Proper Nutrition
-


Mak Environmental Economists Explore Uganda’s Albertine Oil Fields: Identifying Research and Collaboration Opportunities
-


EfD Hosts Policy Dialogue on Energy Efficiency and Reduced Emissions: Hoima Residents Call for Expanded Access to Clean Energy
-


Mak, Oregon State University Sign Cooperation Agreement
-


ENABLING Project Social Scientist Positions: (1) Team Lead (3) Research Associates
Health
ENABLING Project Social Scientist Positions: (1) Team Lead (3) Research Associates
Published
1 week agoon
July 18, 2024By
Mak Editor
Makerere University College of Health Sciences-MAKCHS- Centre of Excellence in Women’s Health in collaboration with Makerere University-Johns Hopkins University (MU-JHU) Care Limited received funding from Bill and Melinda Gates Foundation; Enabling Platforms for Maternal Immunization: Uganda (ENABLING Project). The Project aims to identify, characterize, and support the delivery platform, policy, and preparedness requirements for introducing new maternal vaccines. The Project seeks to recruit suitable candidates for the following positions;
Social Scientist, Team Lead (01)
Social Scientist Research Associate (03)
Duty Station: Kampala
Engagement: Full Time
All applications must be submitted to the email: enablingproject71@gmail.com before Monday, 29th July 2024 at 23:59hrs EAT
Health
Call for Abstracts: Makerere Bioethics Conference 2024
Published
1 week agoon
July 18, 2024By
Mak Editor
The Centre for Bioethics under Makerere University Biomedical Research Centre (MakBRC) is delighted to announce the MAKERERE BIOETHICS CONFERENCE (MakBC 2024), scheduled to take place on 11th and 12th November 2024 at Hotel Africana, Kampala, Uganda. This year’s theme is ‘Contemporary Issues in Bioethics Practice,‘ and we invite researchers, practitioners, and students to submit their abstracts for presentation.
Thematic Areas:
- Emerging Technologies in Health
- Genetics and Genomics
- Assisted Reproductive Health
- Drug and Vaccination Development
- Nanotechnology
- Robotic Surgery
- Data Science
- Artificial Intelligence and Machine Learning
- Biotechnology
- Big Data
- Digital Health
- Research Ethics
- Research Ethics
- Research Integrity
- Clinical Ethics
- Public Health Ethics
Important Dates:
Abstract Submission Deadline: 15th August 2024
Registration Deadline: 16th September 2024
Submission and Registration:
Abstract Submission: Click here to Submit your Abstract
Online Registration: Click here to Register for the Conference
For more information contact Conference Secretariat:
Department of Anatomy,
Last Floor, School of Biomedical Sciences
Makerere University College of Health Sciences,
P.O Box 7072 Kampala, Uganda.
Email: makbioethicsconference@gmail.com
Website: https://chs.mak.ac.ug/makbc2024
Tel: +256 782 363 996 or +256 772 246 681
Health
Mak Researchers Partner with Safe Bangle Technologies to Roll out a Real-Time Domestic Violence Reporting Bracelet
Published
1 week agoon
July 17, 2024By
Mak Editor
By Joseph Odoi
A Consortium of Researchers from Makerere University School of Public Health/Resilient Africa Network (MakSPH/RAN), Medical College of Wisconsin (MCW), Somero Uganda together with Safe Bangle Technologies have rolled out a real time domestic violence reporting bracelet.
This roll out was made possible with support from the United States Agency for International Development (USAID) under the PARTNERSHIPS FOR ENHANCED ENGAGEMENT IN RESEARCH (PEER) program and the National Academies of Sciences.
Dr. Juliet Kiguli, the Principal Investigator from Makerere University, along with Dr. Roy Mayega, Deputy Chief of Party at RAN, and Dr. Agnes Nyabigambo, the study coordinator, initiated the PEER program to identify entry points for testing SafeBangle Technologies (a social enterprise based at Resilient Africa Network (RAN) with a mission to create a safer and more secure environment for women and children through innovative, affordable, and creative technology solutions to curb GBV in Africa.) wearable safety bracelet in the informal settlements. This decision stemmed from findings of increased intimate partner violence (IPV) and gender-based violence (GBV) in three informal settlements in Kampala, Uganda, following a longitudinal study, geospatial mapping, and interviews. The project, titled ‘The Impact of the COVID-19 Pandemic on Gender-Based Violence among Women and Girls in Informal Settlements in Kampala,’ highlighted the urgent need for affordable and immediate reporting mechanisms for violence.”
‘’While carrying out a study after the Covid-19 Pandemic, we identified gaps when it comes to reporting and response to Gender Based Violence (GBV) among women in informal settlements. Therefore, we used incorporated the SafeBangle intervention to solve the problem of lack of affordable and immediate reporting mechanisms for violence using a bracelet that reports violence in real time’’ explained Dr. Kiguli.
Innovation details
According to Saul Kabali and Messach Luminsa, the innovators behind SafeBangle from SafeBangle Technologies, hosted at the Resilient African Network Lab. ‘’The inspiration behind SafeBangle came from a deeply personal place. ‘’We heard countless stories of women who couldn’t call for help during moments of danger. We were deeply affected by the story of Aisha, a young woman in a rural village who was attacked while walking home alone at night. With no way to call for help, she felt helpless and vulnerable. This incident made us realize the critical need for immediate reporting alert tools, accessible to women like Aisha. We knew technology could play a crucial role and this incident awakened a strong desire in us to create a solution’’

“While developing SafeBangle, we tested with the users in both rural and urban contexts. We piloted the innovation around Kampala with support from Digital Human Righs Lab and Naguru Youth Health Network as well as it in five districts of Karamoja region with support from Save the Children and Response Innovation Lab. Right now it has become handy in Kamapala‘s informal settlements. We envision a future where SafeBangle becomes a standard tool in the fight against GBV, ensuring every woman feels safe and secure as it has the potential to transform how we respond to GBV in Africa” added Kabali.
HOW THE SAFEBANGLE TECHNOLOGY WORKS
The SafeBangle is wearable technology similar to a smartwatch that sends an alarm by SMS to people chosen by a woman herself if she feels threatened.

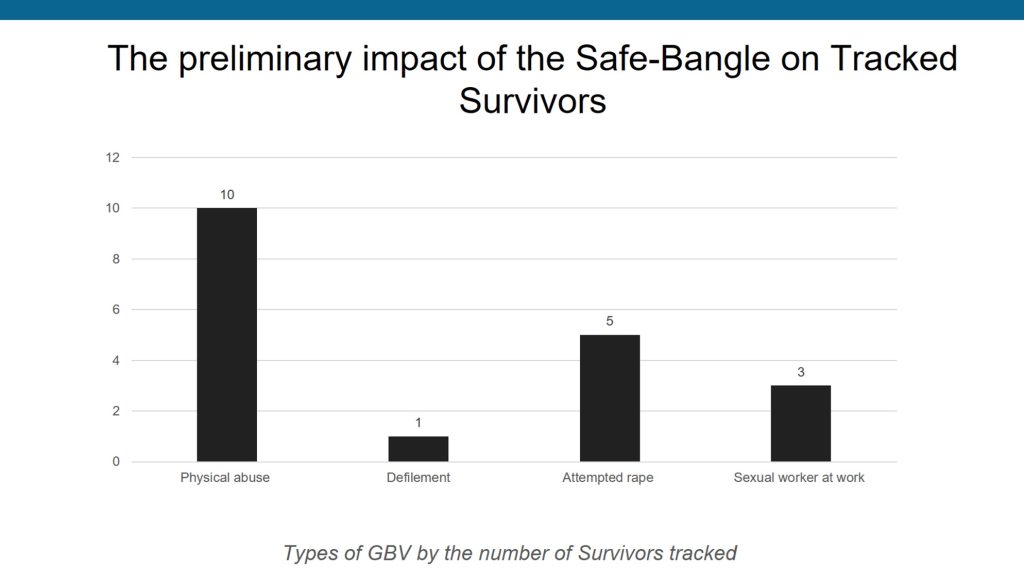
In terms of the acceptability of the SafeBangle innovation as a solution to GBV among at-risk women in informal settlements Of the 72 adolescent girls and women who received the SafeBangle, 22 activated the reporting button, resulting in 19 receiving immediate and appropriate support, including counseling, police intervention, and health services.
All adolescent girls and women who experienced GBV received a phone call from Somero Uganda to discuss the most appropriate intervention, including counseling, police cases being handled by the probation office, referral for health services, and post-exposure prophylaxis. All the GBV survivors received support and are still receiving continuous follow-up.
Researchers conducted a survey among 644 girls and women in Kinawataka (Nakawa Division) and Bwaise (Kawempe Division) to gain insights into awareness and understanding of sexual and gender-based violence among adolescent girls and women in informal settlements. The survey measured socioeconomic factors, mental health symptoms, and exposure to GBV. Focus group interviews were conducted with a separate sample of women over 18 in the settlements to explore responses to GBV.
A tabular representation of the key findings and lessons learned from your study on gender-based violence (GBV)
| Key Findings | Lessons learned |
| Prevalence of GBV. – Overall prevalence: 34.1% of women and girls reported experiencing GBV. – Among adolescents (15-19 years): Over 50% reported experiencing GBV. | – The pandemic highlighted the need for accessible and comprehensive support services for GBV survivors. – Schools emerged as crucial safe spaces for girls, emphasizing their well-being during crises. – Economic independence proved crucial, enabling women to leave abusive environments. – Involving men and boys as allies in GBV prevention efforts is essential. |
| Age-related trends | – GBV prevalence tends to decrease with increasing age. |
| Physical and health consequences. | – Women and girls suffered physical violence, injuries, and deaths, primarily from domestic violence and unsafe abortions due to limited healthcare access. – GBV resulted in unintended pregnancies, unsafe abortions, and increased risk of sexually transmitted diseases (STIs) like HIV/AIDS. |
| Social and economic impact. . | – GBV contributed to family breakups, strained marriages due to financial stress. – Economic hardships forced some women and girls into transactional sex, exposing them to further health risks and exploitation. – Pandemic-related job losses and economic constraints increased financial dependence on abusers, trapping women in violent situations. – School closures and increased household responsibilities limited women’s job opportunities and subjected them to sexual harassment. |
| Psychological effects | – Survivors experienced guilt, shame, anxiety, fear, and suicidal thoughts due to ongoing abuse. |
| Long-term effects | – Post-COVID-19, survivors faced disrupted education, early marriages, pregnancies, social stigma, and persistent mental health issues. |
Reproductive Health Consequences: GBV resulted in unintended pregnancies, unsafe abortions, and increased risk of sexually transmitted diseases (STIs) like HIV/AIDS.
Family Breakdown: The rise in GBV led to family breakups as women fled abusive relationships. Marriages were strained due to increased financial stress.
Transactional Sex for Survival: Desperate for basic needs due to job losses and economic hardship, some women and girls resorted to transactional sex, exposing them to further health risks and exploitation.
One study participant stated, “The time of COVID-19 was so terrible for some of us. We in fact got a lot of diseases from it because you would want to get food and didn’t have money. That way you would be forced to get a man who would use you and pay.” – (FGD_Girls_19–24years_Kinawataka).
Economic Effects: COVID-19 restrictions caused job losses and limited economic opportunities, particularly for women in the informal sector. This increased financial dependence on abusers and trapped women in violent situations.
Limited Access to Employment: School closures and increased household chores limited women’s ability to seek employment, perpetuating gender inequality in the workforce. Some faced sexual harassment from potential employers.
Psychological Effects: Survivors of GBV experienced guilt, shame, anxiety, fear, and even suicidal thoughts due to the constant threat and unpredictability of abuse.
Post-COVID Effects: GBV survivors faced long-term consequences, including disrupted education, early marriage, early pregnancy, social stigma, and persistent mental health issues.
Lessons learned
The pandemic highlighted the need for accessible and comprehensive support services for survivors of GBV, the significance of schools as safe spaces for girls, and the need to prioritize their well-being during crises. Economic empowerment emerged as a significant protective factor for women and girls. Those with greater economic independence were better equipped to leave abusive environments and secure their safety and well-being, while dependent ones suffered abuses. Engaging men and boys as allies in the fight against GBV and involving them in prevention efforts can help promote positive behavior change and foster more equitable relationships.
Recommendations
To address GBV against women and girls, the researchers recommend the following moving forward;
- There is need to integrate technology-driven solutions like SafeBangle into national GBV prevention and response strategies. SafeBangle can be a valuable tool for policymakers as cases of violence that would have gone unreported will be brought to light and the would-be victims will be able to get immediate help from trusted relatives and friends.
- Provide economic opportunities and vocational training for women and girls to enhance their financial independence and reduce vulnerability to violence. There is therefore a need to introduce education and training programs that empower women and girls, by providing them with skills, resources, and opportunities to start their own ventures and to participate fully in community affairs.
- Strengthen and enforce existing laws and policies related to GBV, including laws against domestic violence, child marriage, and sexual assault without discrimination be it for law enforcers, leaders, and employers where such cases were suffocated. Ensure that perpetrators are held accountable through swift and fair legal processes that have no room for corruption.
- Establish and promote effective, accessible, and confidential reporting mechanisms for GBV incidents that provide confidence and can be trusted by survivors to enhance reporting of such incidences of GBV. Community Engagement and Involvement: Involve community leaders, religious leaders, and elders in discussions about GBV to promote gender equality, change social norms, and reinforce the message that violence against women and girls is unacceptable.
- Launch extensive public awareness campaigns to challenge harmful gender norms, report cases of GBV, raise awareness about the consequences of GBV, and promote positive behaviors and attitudes towards women and girls.
- Implement comprehensive sexuality education in schools and communities, educating young people about healthy relationships, consent, and reproductive rights to be able to make informed decisions about their own lives and well-being.
- Engage men and boys as allies in the fight against GBV, encouraging them to challenge harmful masculinity norms and behaviors. This will help minimize GBV because mostly they are the perpetrators. Strengthening Support for Survivors: Provide ongoing support and follow-up services for survivors of GBV mostly counselling services to aid their recovery and facilitate their reintegration into society.
- Provide ongoing support and follow-up services for survivors of GBV, mostly counseling services to aid their recovery and facilitate their reintegration into society.
- Provide avenues to seek free or subsidized services by survivors of GBV medical services and legal processes by survivors of GBV to enhance reporting of GBV cases, access to medical care, counseling, legal support, and other essential services.
- Encourage and support more research and innovations like SafeBangle to curb incidents of GBV.
- A comprehensive and inclusive approach is required. The efforts should involve government institutions, civil society organizations, community leaders, and individuals working together to address the root causes and provide support to survivors.
- Involve media in GBV prevention activities and for enhancing campaigns against GBV mostly on radio and TV.
MORE ABOUT THE STUDY
The core project team, included researchers at Makerere University School of Public Health (MakSPH), Medical College of Wisconsin (MCW) led by Prof. Julia Dickson-Gomez, SafeBangle Technologies, and Somero Uganda, a community-focused NGO, began the project by designing their research protocol and taking a CITI Program course on human subjects social/behavioral research. Team members also met with the Ministry of Gender, Labour, and Social Development (MGLSG) in support of the gender-based violence policy process, Ministry of Health and local government. They also established relationships with the Kampala Capital City Authority (KCCA) and Nakawa and Kawempe probation offices to support legal processes for the GBV survivors. SafeBangle Team also received an award from Defenders Protection Initiative.
Trending
-

 General1 week ago
General1 week agoDiploma/Degree Holders Admission Lists 2024/25
-

 General1 week ago
General1 week agoAdvert: Mature Age Entry Scheme – Private Sponsorship 2024/2025
-

 General5 days ago
General5 days agoProf. Buyinza Mukadasi Appointed Acting DVC Academic Affairs
-

 General2 weeks ago
General2 weeks agoAfrican Futures Research Leadership Program: Cohort 5 – Call for Scholars
-

 General2 weeks ago
General2 weeks agoNow Open: CADFP Project Requests
